With the rollout of COVID-19 vaccines accelerating, people are increasingly asking which vaccine is best?
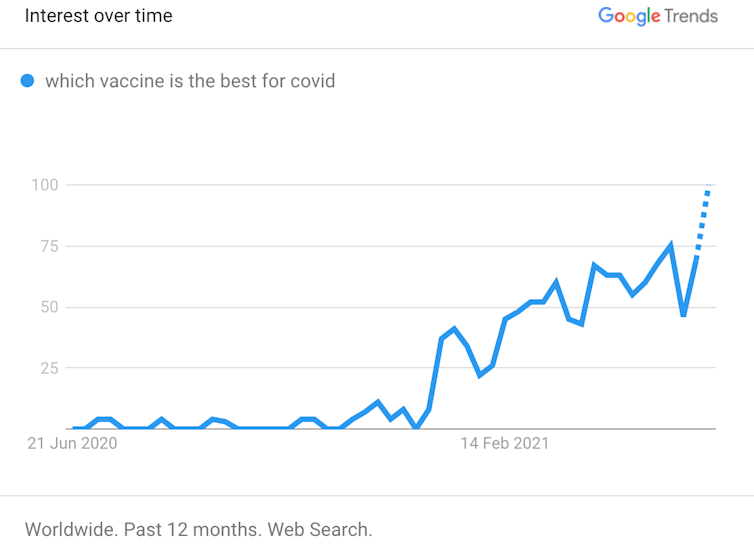
Even if we tried to answer this question, defining which vaccine is “best” is not simple. Does that mean the vaccine better at protecting you from serious disease? The one that protects you from whichever variant is circulating near you? The one that needs fewer booster shots? The one for your age group? Or is it another measure entirely?
Even if we could define what’s “best”, it’s not as if you get a choice of vaccine. Until a suite of vaccines become available, the vast majority of people around the world will be vaccinated with whichever vaccine is available. That’s based on available clinical data and health authorities’ recommendations, or by what your doctor advises if you have an underlying medical condition. So the candid answer to which COVID vaccine is “best” is simply the one available to you right now.
Still not convinced? Here’s why it’s so difficult to compare COVID vaccines.
Clinical trial results only go so far
You might think clinical trials might provide some answers about which vaccine is “best”, particularly the large phase 3 trials used as the basis of approval by regulatory authorities around the world.
These trials, usually in tens of thousands of people, compare the number of COVID-19 cases in people who get the vaccine, versus those who get a placebo. This gives a measure of efficacy, or how well the vaccine works under the tightly controlled conditions of a clinical trial.
And we know the efficacy of different COVID vaccines differ. For instance, we learned from clinical trials that the Pfizer vaccine reported an efficacy of 95% in preventing symptoms, whereas AstraZeneca had an efficacy of 62-90%, depending on the dosing regime.
Read more: How to read results from COVID vaccine trials like a pro
But direct comparison of phase 3 trials is complex as they take place at different locations and times. This means rates of infection in the community, public health measures and the mix of distinct viral variants can vary. Trial participants can also differ in age, ethnicity and potential underlying medical conditions. https://www.youtube.com/embed/BRKZh_RXJC0?wmode=transparent&start=0 It’s tempting to compare COVID vaccines. But in a pandemic, when vaccines are scarce, that can be dangerous.
We might compare vaccines head to head
One way we can compare vaccine efficacy directly is to run head-to-head studies. These compare outcomes of people receiving one vaccine with those who receive another, in the same trial.
In these trials, how we measure efficacy, the study population and every other factor is the same. So we know any differences in outcomes must be down to differences between the vaccines.
For instance, a head-to-head trial is under way in the UK to compare the AstraZeneca and Valneva vaccines. The phase 3 trial is expected to be completed later this year.
How about out in the real world?
Until we wait for the results of head-to-head studies, there’s much we can learn from how vaccines work in the general community, outside clinical trials. Real-world data tells us about vaccine effectiveness (not efficacy).
And the effectiveness of COVID vaccines can be compared in countries that have rolled out different vaccines to the same populations.
For instance, the latest data from the UK show both Pfizer and AstraZeneca vaccines have similar effectiveness. They both reliably prevent COVID-19 symptoms, hospitalisation and death, even after a single dose.
So what at first glance looks “best” according to efficacy results from clinical trials doesn’t always translate to the real world.
What about the future?
The COVID vaccine you get today is not likely to be your last. As immunity naturally wanes after immunisation, periodic boosters will become necessary to maintain effective protection.
There is now promising data from Spain that mix-and-matching vaccines is safe and can trigger very potent immune responses. So this may be a viable strategy to maintain high vaccine effectiveness over time.
In other words, the “best” vaccine might in fact be a number of different vaccines.
Variant viruses have started to circulate, and while current vaccines show reduced protection against these variants, they still protect.
Companies, including Moderna, are rapidly updating their vaccines to be administered as variant-specific boosters to combat this.
So, while one vaccine might have a greater efficacy in a phase 3 trial, that vaccine might not necessarily be “best” at protecting against future variants of concern circulating near you.
The best vaccine is the one you can get now
It is entirely rational to want the “best” vaccine available. But the best vaccine is the one available to you right now because it stops you from catching COVID-19, reduces transmission to vulnerable members of our community and substantially reduces your risk of severe disease.
All available vaccines do this job and do it well. From a collective perspective, these benefits are compounded. The more people get vaccinated, the more the community becomes immune (also known as herd immunity), further curtailing the spread of COVID-19.
The global pandemic is a highly dynamic situation, with emerging viral variants of concern, uncertain global vaccine supply, patchy governmental action and potential for explosive outbreaks in many regions.
So waiting for the perfect vaccine is an unattainable ambition. Every vaccine delivered is a small but significant step towards global normality.
Wen Shi Lee, Postdoctoral researcher, The Peter Doherty Institute for Infection and Immunity and Hyon Xhi Tan, Postdoctoral researcher, The Peter Doherty Institute for Infection and Immunity
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Read more: