The Vaxzevria (AstraZeneca) is no longer an approved COVID-19 vaccine, the Australian Government Department of Health and Aged Care announced last month.
“From Monday 20 March 2023, Vaxzevria (AstraZeneca) is no longer available as an approved COVID-19 vaccine,” the Department of Health website reads.
AstraZeneca had previously been approved for use as a primary course vaccine on 15 February 2021 and as a booster on 8 February 2022. Almost 14 millions doses of the jab have been administered to Australians since its approval.
The Department of Health says that the Pfizer, Moderna and Novavax vaccines were preferred over AstraZeneca for people aged under 60 due to its “higher risk and observed severity of a rare side effect called thrombosis with thrombocytopenia (TTS) in people aged under 60 compared with people aged 60 years or older.” Despite the rare risk of TTS, the AstraZeneca vaccine was still used as a booster in people aged 18 and over.
While the Department of Health maintains that “COVID-19 vaccines are safe and save lives”, they also list several side effects from the jabs. Along with TTS, myocarditis (inflammation of the heart) and pericarditis (inflammation of the membrane around the heart) are also listed as rare complications from the AstraZeneca vaccine. Myocarditis and pericarditis are also listed as rare side effects for the Pfizer, Moderna and Novavax vaccines.
According to the latest safety report from the Therapeutic Goods of Australia (TGA), “to March 19 2023, just under 14 million doses of Vaxzervria (AstraZeneca) have been administered in Australia.”
The safety report also states that there have been 48,721 adverse event reports from the AstraZeneca jab out of a total of 137,970. The Pfizer shot has 81,298 adverse event reports from over 44 million doses. The Moderna vaccine has 7,477 adverse events from more than five million doses. The Novavax jab has 990 adverse events from 247,000 doses.
Change to booster shot guidance
Earlier this year, on 8 February, ATAGI revised their advice for the COVID-19 vaccine booster dose.
A COVID-19 booster shot is now only recommended for those over 65 years of age, and those between 18-64 years of age who have medical comorbidities that increase their risk of severe COVID-19, or disability with significant or complex health needs.
“An additional COVID-19 booster dose is anticipated to address waning of protection against severe COVID-19 prior to winter. This will provide an increase in protection against severe illness and protect the healthcare system during a time of high demand,” it reads.
The new advice recommends against a booster for children and adolescents aged under the age of 18 who do not have any risk factors for severe COVID-19. Those between five and 17 years of age with risk factors and those aged between 18 and 64 with no risk factors are to ‘consider’ a booster shot.
The advice also points to the increased risk of health complications for young people from COVID-19 booster doses.
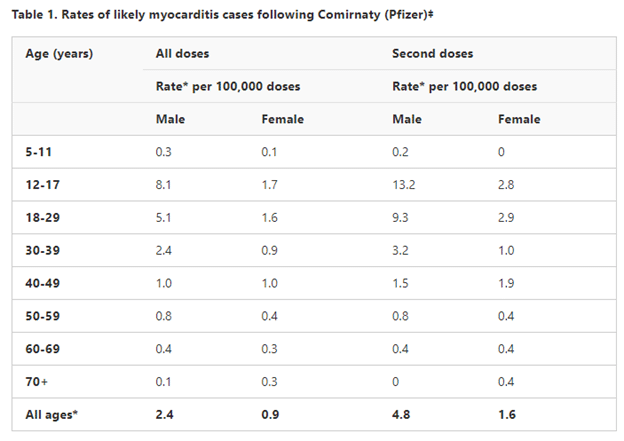
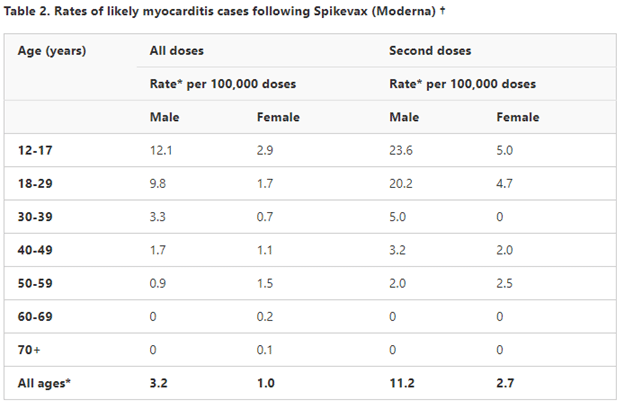
“Adolescents and younger adults have a lower age-related risk of severe COVID-19, and a comparatively higher risk of myocarditis following vaccination. The risk of myocarditis is highest in people aged 16-30 years (peak 16-18 years), and is higher in males than females,” it reads.
The latest safety report from the TGA illustrates the increased risk of myocarditis in young people, particularly males.