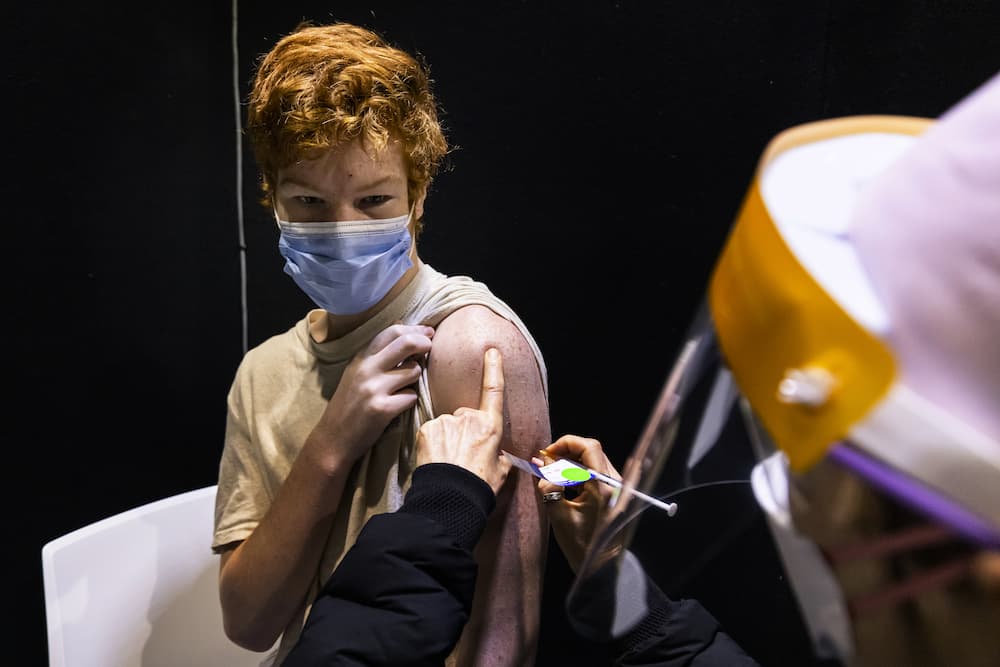
COVID-19 boosters are not required for 12- to 15-year-olds yet, says Australia’s vaccine advisory group.
The call follows a provisional go-ahead for a Pfizer booster for the age group by the Therapeutic Goods Administration (TGA) on Friday.
The medical regulator recommended the cohort receive a third shot six months after their first two regardless of which approved vaccine they had received as their primary course.
The TGA said its review of overseas data in deciding whether to push ahead with the booster had been rigorous and followed approvals issued in Israel, the United Kingdom and the United States.
However the Australian Technical Advisory Group on Immunisation (ATAGI), which is charged with granting final approval, has declined to follow suit.
“ATAGI has reviewed evidence on the benefits and risks of a booster dose of Pfizer COVID-19 vaccine in adolescents in Australia aged 12-15 years,” it said in a statement on its website.
“Current data suggests COVID-related serious illness is very rare in adolescents aged 12-15, particularly after completion of a primary series of COVID-19 vaccination.”
ATAGI said at this time, it does not recommend 12- to 15- year-olds receive the booster and it will meanwhile continue to review international evidence on efficacy.
“ATAGI continues to strongly recommend vaccination of all young people aged 5 to 15 years with two primary doses of a COVID-19 vaccine, including those who may have previously had COVID-19; Three primary doses are recommended for those in this age group who are severely immunocompromised,” it said.
“ATAGI will continue to review and consider new evidence on the benefits and risks of any additional doses in 12-15 year olds, including for those with underlying medical conditions.”
Only Australians aged 16 and over continue to have access to a third jab.
As of Saturday, almost 70 per cent of the eligible population, or more than 13.1 million people in total, had received their booster.
On Monday, the rollout began for a fourth dose – or second booster – for the elderly and vulnerable ahead of winter.
Meanwhile another regulatory body, the Pharmaceutical Benefits Advisory Committee, has recommended the PBS listing of COVID-19 drugs nirmatrelvir and ritonavir.
The former is an oral treatment that inhibits SARS-CoV-2 protein to stop the virus replicating and is used to treat people at high risk of progressing to severe or critical stage.
Ritonavir slows nirmatrelvir’s breakdown to help it remain in the body longer and at higher concentrations.
LATEST 24-HOUR COVID-19 DATA FROM ACROSS AUSTRALIA:
ACT: 959 cases, one death, 62 in hospital, three in ICU
NSW: 17,597 cases, 10 deaths, 1437 in hospital, 47 in ICU
Victoria: 9610 cases, seven deaths, 366 in hospital, 15 in ICU
Queensland: 8687 cases, two deaths, 480 in hospital, 16 in ICU
Western Australia: 6566 cases, three deaths, 236 in hospital, eight in ICU
South Australia: 4777 cases, one death, 201 in hospital, 13 in ICU
Tasmania: 1803 cases, no deaths, 38 in hospital, one in ICU
Northern Territory: 471 cases, no deaths, 24 in hospital, no one in ICU
Canberra Daily would love to hear from you about a story idea in Canberra and the surrounding region. Click here to submit a news tip.